Page 312 - Vitamin D and Cancer
P. 312
13 Vitamin D and Colorectal Cancer 299
laboratories experienced and dedicated to performing these assays when compared
to results obtained from standard hospital clinical chemistry laboratories. A number
of RIA are also available for serum 1, 25-D measurements and have extensive utili-
3
ties in the management of patients with chronic renal diseases and more recently in
cancer patients on calcitriol Phase I/II clinical trials.
More comprehensive and simultaneous analysis of the various serum vitamin D3
metabolites profiles will be needed as our knowledge of the impact of vitamin D3
status on a number of important chronic human diseases expands. The use of new
analytical technologies such as atmospheric pressure chemical ionization (APCI)
with positive ion mode LC/MS/MS method is likely to improve the specificity and
accuracy of the analysis of the serum vitamin D metabolites. At the same time, this
3
new technology can provide comprehensive serum vitamin D metabolites profiles
3
including serum 24,25-D levels that have not been reported in cancer. Our study
3
which utilizes APCI with positive ion mode LC/MS/MS and DiaSorin RIA to mea-
sure serum 25-D levels in colorectal patients receiving 400 and 2,000 IU of oral
3
cholecalciferol daily have confirmed the dose dependency and biphasic character-
istics of the serum 25-D pharmacokinetics. The initial phase of increase in serum
3
25-D levels is approximately 2 months long while the second phase is character-
3
ized by the attainment of a steady state (plateau) serum 25-D levels that lasts as
3
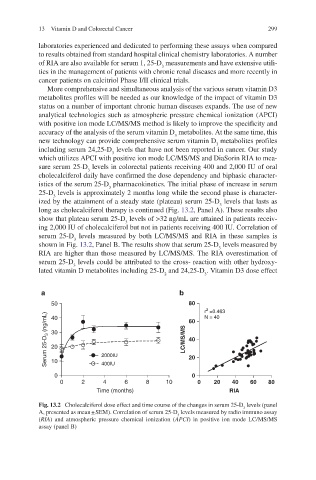
long as cholecalciferol therapy is continued (Fig. 13.2, Panel A). These results also
show that plateau serum 25-D levels of >32 ng/mL are attained in patients receiv-
3
ing 2,000 IU of cholecalciferol but not in patients receiving 400 IU. Correlation of
serum 25-D levels measured by both LC/MS/MS and RIA in these samples is
3
shown in Fig. 13.2, Panel B. The results show that serum 25-D levels measured by
3
RIA are higher than those measured by LC/MS/MS. The RIA overestimation of
serum 25-D levels could be attributed to the cross- reaction with other hydroxy-
3
lated vitamin D metabolites including 25-D and 24,25-D . Vitamin D3 dose effect
2 3
a b
50 80
2
r =0.463
Serum 25-D 3 (ng/mL) 30 2000IU LC/MS/MS 40
40
N = 40
60
20
20
10
0 400IU 0
0 2 4 6 8 10 0 20 40 60 80
Time (months) RIA
Fig. 13.2 Cholecalciferol dose effect and time course of the changes in serum 25-D levels (panel
3
A, presented as mean + SEM). Correlation of serum 25-D levels measured by radio immuno assay
3
(RIA) and atmospheric pressure chemical ionization (APCI) in positive ion mode LC/MS/MS
assay (panel B)