Page 24 - Vitamin D and Cancer
P. 24
1 Vitamin D: Synthesis and Catabolism 11
Other genes modified by epigenetic events could be those coding for the vitamin
D system. Kim et al. [92] demonstrated that the negative response element in the
CYP27B1 promoter is regulated by the ligand-activated vitamin D receptor through
recruitment of histone deacetylase, a critical step for chromatin structure remodel-
ing in suppression of the CYP27B1 gene. In addition, this transrepression by VDR
requires DNA methylation in the CYP27B1 gene promoter. However, this study
was done in kidney cells and not in tumor-derived cells. Another study highlighted
the relevance of different microenvironments (tumor versus normal) for the regula-
tion of CYP24A1: CYP24A1 promoter hypermethylation was present in endothe-
lial cells derived from tumors, but not from normal tissue [93].
In a mouse model of chemically induced colon cancer, protection against tumor
incidence by estrogen was associated with decreased CpG island methylation of the
VDR promoter and enhanced VDR expression [94]. When we tested colon cancer
cell lines derived from moderately differentiated G2 tumors (COGA-1 cells) and
from undifferentiated G3 tumors (COGA-13 cells) for expression of vitamin D
hydroxylases and compared results with the differentiated colon cancer cell line
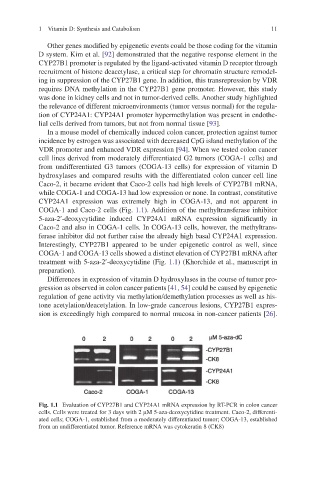
Caco-2, it became evident that Caco-2 cells had high levels of CYP27B1 mRNA,
while COGA-1 and COGA-13 had low expression or none. In contrast, constitutive
CYP24A1 expression was extremely high in COGA-13, and not apparent in
COGA-1 and Caco-2 cells (Fig. 1.1). Addition of the methyltransferase inhibitor
5-aza-2¢-deoxycytidine induced CYP24A1 mRNA expression significantly in
Caco-2 and also in COGA-1 cells. In COGA-13 cells, however, the methyltrans-
ferase inhibitor did not further raise the already high basal CYP24A1 expression.
Interestingly, CYP27B1 appeared to be under epigenetic control as well, since
COGA-1 and COGA-13 cells showed a distinct elevation of CYP27B1 mRNA after
treatment with 5-aza-2¢-deoxycytidine (Fig. 1.1) (Khorchide et al., manuscript in
preparation).
Differences in expression of vitamin D hydroxylases in the course of tumor pro-
gression as observed in colon cancer patients [41, 54] could be caused by epigenetic
regulation of gene activity via methylation/demethylation processes as well as his-
tone acetylation/deacetylation. In low-grade cancerous lesions, CYP27B1 expres-
sion is exceedingly high compared to normal mucosa in non-cancer patients [26].
Fig. 1.1 Evaluation of CYP27B1 and CYP24A1 mRNA expression by RT-PCR in colon cancer
cells. Cells were treated for 3 days with 2 mM 5-aza-deoxycytidine treatment. Caco-2, differenti-
ated cells; COGA-1, established from a moderately differentiated tumor; COGA-13, established
from an undifferentiated tumor. Reference mRNA was cytokeratin 8 (CK8)