Page 302 - Vitamin D and Cancer
P. 302
12 The Vitamin D Signaling Pathway in Mammary Gland and Breast Cancer 289
12.7 Conclusions and Directions for Future Research
In summary, the VDR is expressed in normal mammary epithelial cells, where it
regulates proliferation, apoptosis & differentiation via distinct targets at different
stages of development. In mice, deficiency of the VDR alters glandular growth
during puberty, pregnancy and aging, and enhances risk for mammary gland
transformation. 1,25D and numerous synthetic vitamin D analogs effectively
inhibit growth and induce apoptosis in breast cancer cells & tumors, and these
effects require the VDR. VDR agonists also inhibit growth of normal human
mammary epithelial cells, and evidence suggests that autocrine bio-activation of
vitamin D precursors can occur within mammary cells. Thus, data from both human
tissues and animal models support the concept that the VDR and its ligand induce
a program of gene expression that contributes to maintenance of the differentiated
phenotype in breast cells, a concept which is consistent with a role for vitamin D
in both prevention and treatment of breast cancer. However, the specific mecha-
nisms by which the 1,25D-VDR complex elicits such diverse changes in cell behav-
ior, in particular the relative importance of genomic versus nongenomic mechanisms
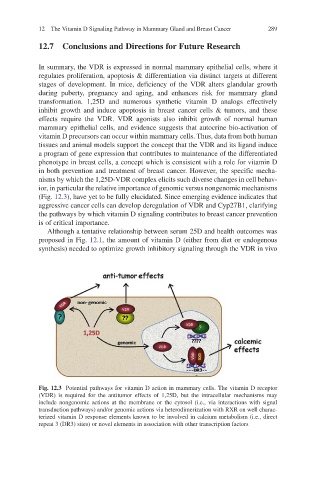
(Fig. 12.3), have yet to be fully elucidated. Since emerging evidence indicates that
aggressive cancer cells can develop deregulation of VDR and Cyp27B1, clarifying
the pathways by which vitamin D signaling contributes to breast cancer prevention
is of critical importance.
Although a tentative relationship between serum 25D and health outcomes was
proposed in Fig. 12.1, the amount of vitamin D (either from diet or endogenous
synthesis) needed to optimize growth inhibitory signaling through the VDR in vivo
Fig. 12.3 Potential pathways for vitamin D action in mammary cells. The vitamin D receptor
(VDR) is required for the antitumor effects of 1,25D, but the intracellular mechanisms may
include nongenomic actions at the membrane or the cytosol (i.e., via interactions with signal
transduction pathways) and/or genomic actions via heterodimerization with RXR on well charac-
terized vitamin D response elements known to be involved in calcium metabolism (i.e., direct
repeat 3 (DR3) sites) or novel elements in association with other transcription factors